Carbohydrates:Nature's Most Abundant Organic Substance
Carbohydrates are macromolecules.Carbohydrates are compounds of carbon, hydrogen, and oxygen. These elements usually occur in the ratio of 1 C: 2 H:1 O
and are grouped as H --C --OH.
Carbohydrates were defined as 'hydrates of carbon' because hydrogen and oxygen were present in ratio of water as 2H:1O like C6H12O6. But later on it was found that most of the carbohydrates have hydrogen and oxygen are not in ratio of water and some of the compounds have hydrogen and oxygen in ratio of water, are not even carbohydrates. The modern definition of carbohydrates is "the polyhydroxy compounds of aldehydes and ketones are called carbohydrates"
Carbohydrates are a loosely defined group of molecules that all
contain carbon, hydrogen, and oxygen in the molar ratio 1:2:1.
Their empirical formula is (CH2O)n, where n is the number of carbon atoms. Because they contain many carbon–hydrogen
(C—H) bonds, which release energy when oxidation occurs, carbohydrates are well suited for energy storage. Sugars are among
the most important energy-storage molecules, and they exist in
several different forms. Carbohydrates function in
protoplasm(protoplasm is the content of the cell including the cell membrane, cytoplasm of the cell and the nucleus (nuclear membrane and nucleoplasm) of the cell) mainly as structural elements and as a source of
chemical energy. Glucose is the most important of these energy storing carbohydrates. Familiar examples of carbohydrates
include sugars, starches, and cellulose (the woody structure of
plants). Cellulose occurs on earth in greater quantities than all
other organic materials combined. Carbohydrates are synthesized by green plants from water and carbon dioxide, with the
aid of solar energy. This process, called photosynthesis, is a
reaction upon which all life depends, for it is the starting point
in the formation of food.
Types of Carbohydrates
There are three main groups of carbohydrates
1.Monosaccharides/Simple Sugars
2.Disaccharides/Double Sugars
3.Polysaccharides/Complex Sugars
1.Monosaccharides
The simplest of the carbohydrates are monosaccharides.(Greek mono, “single,” and Latin saccharum, “sugar”).They generally contain carbon number from 3 to 10,but above 7 are not biologically important. Their empirical formula is multiple of CH2O.
 )group are called 'Aldose',while containing ketone (
)group are called 'Aldose',while containing ketone ( ) group are called 'Ketose'.e. g glucose is an aldose while fructose is a ketose.
) group are called 'Ketose'.e. g glucose is an aldose while fructose is a ketose.
Important Properties
Monosaccharides are sweet in taste.
They can form 'true solution' as they are easily soluble in water.
They are 'non-hydrolysable'.They can not be hydrolysed into simple sugars.
In aqueous solution(the solution in which the solvent is water) ,most monosaccharides for ring structure.
Monosaccharides are classified into two classes on the basis of functional group which they have, either 'Aldehyde or Ketone group. Monosaccharides with aldehyde ( )group are called 'Aldose',while containing ketone (
)group are called 'Aldose',while containing ketone ( ) group are called 'Ketose'.e. g glucose is an aldose while fructose is a ketose.
) group are called 'Ketose'.e. g glucose is an aldose while fructose is a ketose. Types of Monosaccharides
Monosaccharides have following types on the basis of number of carbons from 3 to 7.
1.Trioses
Monosaccharides with 3 carbons are called trioses.formula,C3 H6 O3 Two trioses are
2.Dihydroxyacetone or Glycerone (ketose)
2.Tetroses
3.Pentoses
Monosaccharides with 5 carbons are called pentoses. C5 H10 O5 e. g Ribose
Ribose is an Aldo sugar. It gives five corner ring structure in solution form. That ring is called Ribofuranose.it present in RNA
When we remove an oxygen atom from carbon no. 2 it becomes deoxyribose. It is also a pentose sugar. It is present in DNA.
Ribose is an Aldo sugar. It gives five corner ring structure in solution form. That ring is called Ribofuranose.it present in RNA
When we remove an oxygen atom from carbon no. 2 it becomes deoxyribose. It is also a pentose sugar. It is present in DNA.
4.Hexoses
Monosaccharides with 6 carbons are called hexoses. C6 H12 O6. e.g glucose, galactose, fructose, manose.
The empirical
formula of 6-carbon sugars is
C6H12O6 or (CH2O)6
Six-carbon sugars can exist as a straight chain, but dissolved in
water (an aqueous environment) they almost always form rings.
monosaccharides for energy storage is glucose.
Glucose has seven energy-storing C—H bonds
(figure below) . Depending on the orientation of
the carbonyl group (C O) when the ring is
closed, glucose can exist in two different forms:
alpha (α) or beta (β).
Glucose is a linear 6-carbon
molecule that forms a six-membered ring in solution. Ring closure occurs such that two
forms can result: α-glucose or α-glucopyranose and β-glucose or β-glucopyranose. These structures differ only in the position
of the —OH bound to carbon 1.
The empirical
formula of 6-carbon sugars is
C6H12O6 or (CH2O)6
Six-carbon sugars can exist as a straight chain, but dissolved in
water (an aqueous environment) they almost always form rings.
Glucose (also
called dextrose)
The most important of the 6-carbonmonosaccharides for energy storage is glucose.
Glucose has seven energy-storing C—H bonds
(figure below) . Depending on the orientation of
the carbonyl group (C O) when the ring is
closed, glucose can exist in two different forms:
alpha (α) or beta (β).
Glucose is a linear 6-carbon
molecule that forms a six-membered ring in solution. Ring closure occurs such that two
forms can result: α-glucose or α-glucopyranose and β-glucose or β-glucopyranose. These structures differ only in the position
of the —OH bound to carbon 1.
Sugar isomers have structural differences
Glucose is not the only sugar with the formula
C6H12O6. Both structural isomers and stereoisomers of this simple 6-carbon skeleton exist
in nature. Fructose is a structural isomer that
differs in the position of the carbonyl carbon
(C O); galactose is a stereoisomer that differs
in the position of —OH and —H groups relative to the ring (figure ). These differences
often account for substantial functional differences between the isomers. Your taste buds can
discern them: Fructose tastes much sweeter
than glucose, despite the fact that both sugars
have identical chemical composition. Enzymes that act on different sugars can distinguish both the structural isomers and stereoisomers of this basic 6-carbon skeleton. The different stereoisomers of glucose are also important in the polymers
that can be made using glucose as a monomer, as you will see later
in this section.
Structural isomers and stereoisomers. The
sugars glucose, fructose, and galactose are isomers with the
empirical formula C6H12O6. A structural isomer of glucose,
such as fructose, has identical chemical groups bonded
to different carbon atoms. A stereoisomer of glucose, such
as galactose, has identical chemical groups bonded to the
same carbon atoms but in different orientations (the —OH at
carbon 4).
The “chair” diagram
( Figure below) of glucose best represents its true configuration, but
all forms of glucose, however represented, are chemically equivalent.
5.Heptoses
Monosaccharides with 7 carbons are called heptoses. C7 H14 O7.e.g Sedoheptulose
2.Disaccharides
Disaccharides
(Greek di,
“two”)
are double sugars formed by bonding two
simple sugars.
"The sugars composed of two monosaccharides are called disaccharides".
Important Properties
Disaccharides are both less sweet in taste and less soluble in water.
They are hydrolysable,they yield 2 monosaccharides on hydrolysis.
Their general formula is C12 H22 O11.
Examples
Both animals and plants transport sugars within their bodies. In
humans, the glucose that circulates in the blood does so as a simple monosaccharide. In plants and many other animals, however,
glucose is converted into a transport form before it is moved from
place to place within the body. In such a form, it is less readily
metabolized during transport.Transport forms of sugars are commonly made by linking
two monosaccharides together to form a disaccharide (Greek di,
“two”). Disaccharides serve as effective reservoirs of glucose
because the enzymes that normally use glucose in the organism
cannot break the bond linking the two monosaccharide subunits.
Enzymes that can do so are typically present only in the tissue
destined to use the glucose.
There are three important disaccharides.
1.Sucrose(Cane Sugar)
Glucose forms a variety of transport disaccharides. In plants,
glucose instead forms a disaccharide with its structural isomer
fructose. The resulting disaccharide is sucrose, ordinary cane or table sugar
(figure below). Sucrose is the form most plants use to transport
glucose, and it is the sugar that most humans eat.
2.Lactose(milk sugar)
In mammals, glucose is linked to its stereoisomer galactose, forming the disaccharide lactose, or milk sugar. Many
mammals supply energy to their young in the form of lactose.
Adults often have greatly reduced levels of lactase, the enzyme
required to cleave lactose into its two monosaccharide components, and thus they cannot metabolize lactose efficiently. This
effectively reserves the energy stored in lactose for the
offspring.
3.Maltose(malt sugar)
An example is maltose (malt sugar), composed
of two glucose molecules. As shown in Figure below , the two glucose molecules are joined by removing a molecule of water,
causing the sharing of an oxygen atom by the two sugars. All
disaccharides are formed in this manner.
humans, the glucose that circulates in the blood does so as a simple monosaccharide. In plants and many other animals, however,
glucose is converted into a transport form before it is moved from
place to place within the body. In such a form, it is less readily
metabolized during transport.Transport forms of sugars are commonly made by linking
two monosaccharides together to form a disaccharide (Greek di,
“two”). Disaccharides serve as effective reservoirs of glucose
because the enzymes that normally use glucose in the organism
cannot break the bond linking the two monosaccharide subunits.
Enzymes that can do so are typically present only in the tissue
destined to use the glucose.
There are three important disaccharides.
1.Sucrose(Cane Sugar)
Glucose forms a variety of transport disaccharides. In plants,
glucose instead forms a disaccharide with its structural isomer
fructose. The resulting disaccharide is sucrose, ordinary cane or table sugar
(figure below). Sucrose is the form most plants use to transport
glucose, and it is the sugar that most humans eat.
2.Lactose(milk sugar)
In mammals, glucose is linked to its stereoisomer galactose, forming the disaccharide lactose, or milk sugar. Many
mammals supply energy to their young in the form of lactose.
Adults often have greatly reduced levels of lactase, the enzyme
required to cleave lactose into its two monosaccharide components, and thus they cannot metabolize lactose efficiently. This
effectively reserves the energy stored in lactose for the
offspring.
3.Maltose(malt sugar)
An example is maltose (malt sugar), composed
of two glucose molecules. As shown in Figure below , the two glucose molecules are joined by removing a molecule of water,
causing the sharing of an oxygen atom by the two sugars. All
disaccharides are formed in this manner.
3.Polysaccharides
The polymers of many monosaccharides are called polysaccharides.
Polysaccharides are longer sugar polymers made up of monosaccharides that have been joined through dehydration
reactions. Polysaccharides are composed of many molecules of simple
sugars (usually glucose) linked in long chains called polymers.
Their empirical formula is usually written
(C6 H10 O5)n, where n designates the number of simple-sugar subunits in the polymer.
reactions. Polysaccharides are composed of many molecules of simple
sugars (usually glucose) linked in long chains called polymers.
Their empirical formula is usually written
(C6 H10 O5)n, where n designates the number of simple-sugar subunits in the polymer.
Important Properties
They are tasteless and sparingly soluble in water. Polysaccharides are most abundant in nature.
In polysaccharides, few hundred to many thousand monosaccharides are linked together by glycosidic linkage.
Types of polysaccharides
Biologically important polysaccharides are
Starch, glycogen, cellulose, chitin, dextrin, pectin and agar etc.
1.Starch
Organisms store the metabolic energy contained in monosaccharides by first converting them into disaccharides, such as maltose. These are then linked together into insoluble storage
polysaccharides called starches (figure below).
Types of starch
Starches differ mainly in how the long-chain polymers
branch.
1.Amylose Starch The starch with simplest structure is called amylase.
It is composed of many hundreds of α-glucose molecules linked
together in long, unbranched chains. Each linkage occurs between
the carbon 1 (C-1) of one glucose molecule and the C-4 of another,
making them α-1→4 linkages (figure below) The long chains tend
to coil up in water, a property that renders amylose insoluble.
Potato starch is about 20% amylose.
2.Amylopectin
Most plant starch, including the remaining 80% of potato
starch, is a somewhat more complicated variant of amylose called
amylopectin (figure below). Pectins are branched polysaccharides
with the branches occurring at bonds between the C-1 of one molecule and the C-6 of another (α-1→6 linkages). These short
branches consist of 20 to 30 glucose subunits.
2.Glycogen(animal starch)
The molecule comparable to starch in animals is glycogen.
Like amylopectin, glycogen is an insoluble polysaccharide
containing branched amylose chains. Glycogen has a much longer
average chain length and more branches than plant starch .
Glycogen is a polymer of glucose. It is more extensively branched than amylopectin of plants. It is chief storage compound in animals.Glycogen is an important polymer for
storing sugar in animals. It is stored mainly in liver and muscle
cells in vertebrates. When needed, glycogen is converted to glucose and delivered by blood to the tissues. It gives red colour with iodine.
3.Cellulose,the principal structural polysaccharide(Carbohydrate) in plants
Although some chains of sugars store energy, others serve as
structural material for cells. For glucose molecules to link
together in a chain, the glucose subunits must be of the same
form. Starches are α-glucose chains. Cellulose is a β-glucose
chain (figure 3.9a). The bonds between adjacent glucose molecules in cellulose still extend between the C-1 of the first glucose
and the C-4 of the next glucose, but in cellulose these are both
β-1→4 linkages.
The properties of a β-glucose chain are very different
from those of starch. Long, unbranched β-linked chains make
tough fibers. Cellulose is the chief component of plant
cell walls . It is chemically similar to amylose,
with one important difference: The starch-hydrolyzing
enzymes that occur in most organisms cannot break the
bond between two β-glucose units, because they recognize only
α linkages.
Because cellulose cannot be broken down readily by most
creatures, it works well as a biological structural material. But
some animals, such as cows, are able to break down cellulose by
means of symbiotic bacteria and protists in their digestive tracts.
These organisms provide the necessary enzymes for cleaving the
β-1→4 linkages, thus releasing glucose for further metabolism.
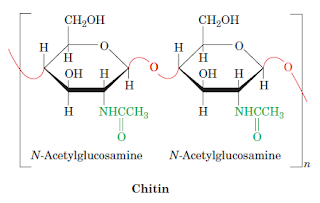
4.Chitin,a structural polysaccharide
Chitin, the structural material found in arthropods and many
fungi, is a polymer of N-acetylglucosamine, a derivative of glucose. When cross-linked by proteins, it forms a tough, resistant
surface material that serves as the hard exoskeleton of insects and
crustaceans (figure below). Few animals are able to digest chitin in