"Proteins are long and linear polymers of amino acids".
The word "protein" is derived from a Greek word "proteios" meaning 'primary', in the lead, or " standing in front.
Proteins are composed of carbon, hydrogen, nitrogen, oxygen and sometimes sulphur.
Amino Acids
"Amino acids are building blocks of proteins".
An amino acid is a compound that contains both a carboxyl group and an amino group.
Although many types of amino acids are known, the α-amino acids(An amino acid in which the
amino group is on the carbon
adjacent to the carboxyl
group) are the most significant in the biological world because they are the monomers from which proteins are
constructed. Amino acids, as their name suggests, contain an amino
group (—NH2) and an acidic carboxyl group (—COOH). The specific order of amino acids determines the protein’s structure and
function. Many scientists believe amino acids were among the
first biologically important molecules formed on the early Earth.
It seems highly likely that the oceans that existed early in the history of the Earth contained a wide variety of amino acids.
Amino acid structure
The generalized structure of an amino acid is shown here as amino
and carboxyl groups bonded to a central carbon atom, with an
additional hydrogen and a functional side group indicated by R(also called side chain) .
These four components completely occupy the four valence bonds
of the central tetrahedral carbon that is called α-carbon.
The unique character of each amino acid is determined by
the nature of the R group. Notice that unless the R group is an H
atom, as in glycine,
amino acids are chiral and can exist as two
enantiomeric forms: d or l. In living systems, only the l-amino
acids are found in proteins.
The R group also determines the chemistry of amino acids.
Thus, serine,
in which the R group is —CH2OH, is a polar molecule. By contrast, alanine,
which has —CH3 as its R group, is non polar.
20 common amino acids are grouped into five chemical
classes, based on their R group.
Note:We usually write the structures of amino acids as neutral ,it is not accurate because it shows an acid (!COOH) and a base (!NH2)
within the same molecule. These acidic and basic groups react with each other to
form a dipolar ion or internal salt. The internal salt of an amino acid is
given the special name zwitterion.Note that a zwitterion has no net charge; it contains one positive charge and one negative charge.Their actual
structure is ionic and depends on pH. In general, amino acids undergo an intramolecular acid–base reaction and form a dipolar ion, or zwitterion as in figure below.
At room temperature this equilibrium lies far to the right. Since one side of the dipolar ion is positively charged and the other negatively charged, amino acids are highly
polar and soluble in water but insoluble in nonpolar organic solvents such as ether and
hydrocarbon solvents. Because they exist as zwitterions, amino acids have many of the properties
associated with salts. They are crystalline solids with high melting points (usually 7200 °C).
In addition, the intramolecular acid–base reaction makes amino acids less acidic and less
basic than most carboxylic acids and amines, respectively.
1.Non-polar amino acids: Non polar amino acids, such as leucine, often have non polar R groups
that contain —CH2 or —CH3.Non polar amino acids are also called hydrophobic amino acids.
 |
| Nonaromatic |
2.Polar uncharged amino acids: Polar uncharged amino acids, such as threonine, have R
groups that contain oxygen (or —OH).
 |
| Nonaromatic |
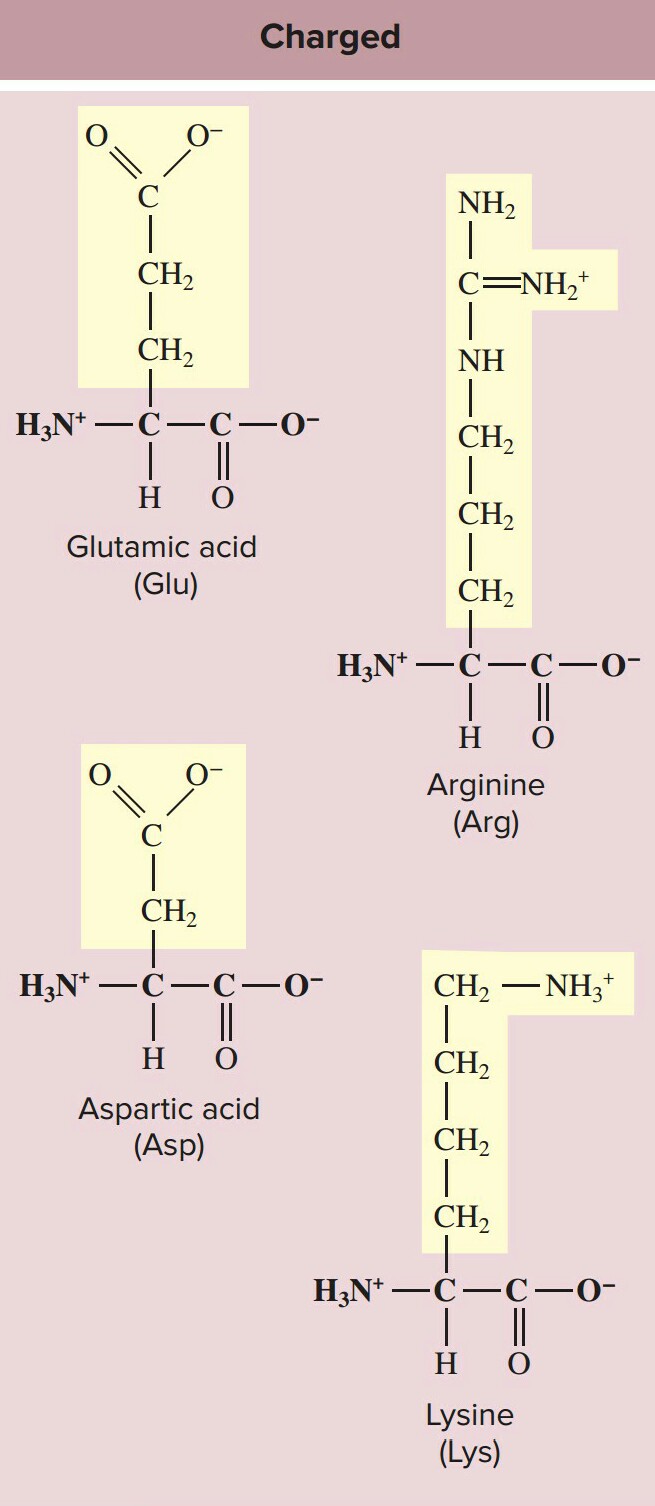
3.Polar charged amino acids:polar charged amino acids, such as glutamic acid, have R groups
that contain acids(acidic amino acids,side chains are negatively charged e.g aspartic acid )
or bases(basic amino acids,side chains are positively charged e.g lysine,since it contains nitrogen)
that can ionize.Polar amino acids are also called hydrophilic amino acids.
 |
| Nonaromatic |
groups that contain an organic (carbon) ring with alternating single and double bonds. These are quite nonpolar.
 |
| Polar charged |
 |
| Polar uncharged |
 |
| Non-Polar |
5.Amino acids with unique properties: Some amino acids have unique properties. Some examples
are methionine, which is often the first amino acid in a
chain of amino acids; proline, which causes kinks in chains;
 |
| Non-Polar |
 |
| Non-Polar |
 |
| Polar uncharged |
Each amino acid affects the shape of a protein differently,
depending on the chemical nature of its side group. For example,
portions of a protein chain with numerous nonpolar amino acids
tend to fold into the interior of the protein by hydrophobic exclusion (more on this later in this section).
Some Other Common l-Amino Acids
Although the vast majority of plant and animal proteins are constructed from just
these 20 a-amino acids, many other amino acids are also found in nature. Ornithine
and citruline, for example, are found predominantly in the liver and are an integral
part of the urea cycle, the metabolic pathway that converts ammonia to urea.
Thyroxin and triiodothyroxin, two of several hormones derived from the amino
acid tyrosine, are found in thyroid tissue. Their principal function is to stimulate metabolism in other cells and tissues.
4-Aminobutanoic acid is found in high concentration (0.8 mM ) in the brain
but in no significant amounts in any other mammalian tissue. It is synthesized in
neural tissue by decarboxylation of the a-carboxyl group of glutamic acid and is a
neurotransmitter in the central nervous system of invertebrates and humans.
Only l-amino acids are found in proteins, and only rarely are d-amino acids a part
of the metabolism of higher organisms. Several d-amino acids, however, along with
their l-enantiomers, are found in lower forms of life. d-Alanine and d-glutamic acid,
for example, are structural components of the cell walls of certain bacteria. Several
d-amino acids are also found in peptide antibiotics.
Peptide bond formation In 1902, Emil Fischer proposed that proteins are long chains of amino acids joined
by amide bonds between the a-carboxyl group of one amino acid and the a-amino
group of another. For these amide bonds, Fischer proposed the special name peptide bond.
In addition to its R group, each amino acid is ionized at physiological pH, with a positive amino (NH3
+) group at one end and a negative carboxyl (COO–
) group at the other. The amino groups on one
amino acid can undergo a dehydration reaction with the carboxyl
group on another amino acid to form a covalent bond. "The covalent bond that links two amino acids is called a peptide bond(The special name given to the
amide bond formed between
the a-amino group of one
amino acid and the a-carboxyl
group of another amino acid).
(figure)
dipeptide serylalanine.
 |
| A tripeptide of valine, cysteine and phenylalanine. |
has a partial double-bond character. This is different from the
N—C and C—C bonds to the central carbon of the amino acid.
This lack of rotation about the peptide bond is one factor that
determines the structural character of the coils and other regular
shapes formed by chains of amino acids.
Peptide is the name given to a short polymer of amino acids. Peptides are classified
by the number of amino acid units in the chain. A molecule containing two amino acids
joined by an amide bond is called a dipeptide. Those containing 3 to 10 amino acids
are called tripeptides, tetrapeptides, pentapeptides, and so on. Molecules containing
more than 10 but fewer than 20 amino acids are called oligopeptides. Those containing several dozen or more amino acids are called polypeptides. Functional proteins usually contain one or more polypeptide chains, with each chain
consisting of hundreds or even thousands of amino acids joined by peptide bonds. By convention, polypeptides are written from the left beginning with the amino
acid having the free —NH3+
group and proceeding to the right toward the amino
acid with the free —COO^ group. The amino acid with the free NH3+
group is
called the N-terminal amino acid (The amino acid at the end of a
polypeptide chain having the
free !NH3
1 group.), and the amino acid with the free !COO2 group
is called the C-terminal amino acid(The amino acid at the end of a
polypeptide chain having the
free !COO2 group). Notice the repeating pattern in the peptide
chain of N-a-carbon-carbonyl, and so on.
Proteins are biological
macromolecules of molecular weight 5000 or greater consisting of one or more polypeptide chains. The distinctions in this terminology are not precise.A protein is composed of one or more long, unbranched
chains of amino acids linked by peptide bonds. Each chain is
called a polypeptide. The terms protein and polypeptide tend to
be used loosely and interchangeably, and this may create confusion. For proteins that include only a single polypeptide chain, the
two terms are synonymous.
The pioneering work of Frederick Sanger in the early 1950s
provided the evidence that each kind of protein has a specific
amino acid sequence. Using chemical methods to remove successive amino acids and then identify them, Sanger succeeded in
determining the amino acid sequence of insulin. In so doing he
demonstrated clearly that this protein has a defined sequence,
which is the same for all insulin molecules. Although many different amino acids occur in nature, only 20 commonly occur in proteins. Above mentioned Figures illustrates these 20 amino acids and their side groups.
Four Levels of Protein Structure
The structure of proteins is usually discussed in terms of a hierarchy of four levels: primary, secondary, tertiary, and quaternary. We will examine this view and then integrate it with
a more modern approach arising from our increasing knowledge
of protein structure.
Primary Structure:Amino acid sequence
"The linear sequence of amino acids in the polypeptide chain" The primary structure of a protein is its amino acid sequence.
Because the R groups that distinguish the amino acids play no role
in the peptide backbone of proteins, a protein can consist of any
sequence of amino acids. Thus, because any of 20 different amino
acids might appear at any position, a protein containing 100 amino
acids could form any of 20^100 different amino acid sequences. This
important property of proteins permits great diversity.The primary structure is like order of letters of along word. The sequence of amino acids in protein is specific. Slight change in primary structure can affect protein conformation and ability to function.
Consider the protein hemoglobin, is a protein that transports oxygen in the blood. It is composed of four polypeptide chains.Hemoglobin is composed of two α-globin
peptide chains and two β-globin peptide chains , made up of a total of 574 amino
acid units.α-globin chains differ from the β-globin chains in the sequence of The
amino acids. Furthermore, any alteration in the identity of any
amino acid in either of the two types of globin chains—even a
single amino acid—can have drastic effects on how the protein functions . If valine is replaced by glutamic acid in just one position on two of these chains, the disease known as sickle-cell anemia results. The red blood cells of people with sickle-cell
anemia take on a sickle shape that impedes circulation, causing damage to major organs. In
the past, sickle-cell anemia was fatal, often resulting in death before age 30—all due to a
change in a few atoms of two amino acids out of 574. Modern therapies have extended the
life span of people with sickle-cell anemia so that they now live into their 40s and 50s.the protein your blood
uses to transport oxygen. (figures).
 |
| The genetic disease known as sickle-cell anemia results in red blood cell with a sickle shape.These cells impede circulation of blood,causing damage to major organs. |
Lysozyme is an antibacterial enzyme that is naturally present in tears, saliva and other body secretions.It is a small protein, composed of only 129 amino acid.
Secondary structure:Hydrogen bonding pattern
"The structure formed by folding or coiling of polypeptide chain with the help of hydrogen bonding is called secondary structure".
Secondary (2°) structure refers to ordered arrangements (conformations) of
amino acids in localized regions of a polypeptide or protein molecule. The first
studies of polypeptide conformations were carried out by Linus Pauling and Robert Corey beginning in 1939. They assumed that in conformations of greatest stability,
all atoms in a peptide bond lie in the same plane and there is hydrogen bonding
between the N—H
of one peptide bond and the C=O
of another, as shown in
Figure
On the basis of model building, Pauling proposed that two types of secondary
structure should be particularly stable: the a-helix and the antiparallel b-pleated
sheet. X-ray crystallography has validated this prediction completely.
The α-Helix
"A delicate coil of perspective chain held to get her by hydrogen bonding between every fourth peptide bond is called α-helix.
In an a-helix pattern shown in Figure 27.14, a polypeptide chain is coiled in a spiral.
As you study this section of a-helix, note the following:
1.The helix is coiled in a clockwise, or right-handed, manner. Right-handed means
that if you turn the helix clockwise, it twists away from you. In this sense, a right handed helix is analogous to the right-handed thread of a common wood or
machine screw.
2.There are 3.6 amino acids per turn of the helix.
3.Each peptide bond is trans and planar.
4.The N—H group of each peptide bond points roughly downward, parallel to the
axis of the helix, and the C=O of each peptide bond points roughly upward, also
parallel to the axis of the helix.
5.The carbonyl group of each peptide bond is hydrogen-bonded to the N—H group
of the peptide bond four amino acid units away from it. Hydrogen bonds are
shown as doted lines.
All R— groups point outward from the helix.
Almost immediately after Pauling proposed the a-helix conformation, other researchers proved the presence of a-helix conformations in keratin, the protein of hair
and wool. It soon became obvious that the a-helix is one of the fundamental folding
patterns of polypeptide chains.
The β-Pleated Sheet"A sheet of polypeptide chain formed by the folding back and forth of polypeptide chain is called β-pleated sheet.
A second common pattern in the secondary structure of proteins is the
β-pleated sheet (Figure).
In this structure, the chain is extended (as opposed
to coiled) and forms a zigzag pattern. The peptide backbones of neighboring chains
interact with one another through hydrogen bonding to form zigzag-shaped sheets.An antiparallel b-pleated sheet consists of extended polypeptide chains with
neighboring chains running in opposite (antiparallel) directions. In a parallel
b-pleated sheet, the polypeptide chains run in the same direction. Unlike the a-helix
arrangement, N—H and C=O groups lie in the plane of the sheet and are roughly
perpendicular to the long axis of the sheet. The C=O group of each peptide bond
is hydrogen-bonded to the N—H group of a peptide bond of a neighboring chain. Figure
As you study this section of b-pleated sheet, note the following:
1.The three polypeptide chains lie adjacent to each other and run in opposite (antiparallel) directions.
2. Each peptide bond is planar, and the a-carbons are trans to each other.
3.The C=O and N—H groups of peptide bonds from adjacent chains point at each
other and are in the same plane so that hydrogen bonding is possible between
adjacent polypeptide chains.
4. The R-groups on any one chain alternate, first above and then below the plane of
the sheet and so on.
The b-pleated sheet conformation is stabilized by hydrogen bonding between
N—H groups of one chain and C=O groups of an adjacent chain. By comparison,
the a-helix is stabilized by hydrogen bonding between N—H and C=O groups
within the same polypeptide chain.
Some proteins—such as silk—have the β-pleated sheet structure throughout their
entire chains. Since the protein chains in the β-pleated sheet are fully extended, silk is
inelastic. Many proteins have some sections that are β-pleated sheet, other sections
that are a-helical, and still other sections that have less regular patterns referred to as
random coils.
Secondary structure:Hydrogen bonding pattern
"The structure formed by folding or coiling of polypeptide chain with the help of hydrogen bonding is called secondary structure".
Secondary (2°) structure refers to ordered arrangements (conformations) of
amino acids in localized regions of a polypeptide or protein molecule. The first
studies of polypeptide conformations were carried out by Linus Pauling and Robert Corey beginning in 1939. They assumed that in conformations of greatest stability,
all atoms in a peptide bond lie in the same plane and there is hydrogen bonding
of one peptide bond and the C=O
Figure
On the basis of model building, Pauling proposed that two types of secondary
structure should be particularly stable: the a-helix and the antiparallel b-pleated
sheet. X-ray crystallography has validated this prediction completely.
The α-Helix
"A delicate coil of perspective chain held to get her by hydrogen bonding between every fourth peptide bond is called α-helix.
In an a-helix pattern shown in Figure 27.14, a polypeptide chain is coiled in a spiral.
As you study this section of a-helix, note the following:
1.The helix is coiled in a clockwise, or right-handed, manner. Right-handed means
that if you turn the helix clockwise, it twists away from you. In this sense, a right handed helix is analogous to the right-handed thread of a common wood or
machine screw.
2.There are 3.6 amino acids per turn of the helix.
3.Each peptide bond is trans and planar.
4.The N—H group of each peptide bond points roughly downward, parallel to the
axis of the helix, and the C=O of each peptide bond points roughly upward, also
parallel to the axis of the helix.
5.The carbonyl group of each peptide bond is hydrogen-bonded to the N—H group
of the peptide bond four amino acid units away from it. Hydrogen bonds are
shown as doted lines.
All R— groups point outward from the helix.
Almost immediately after Pauling proposed the a-helix conformation, other researchers proved the presence of a-helix conformations in keratin, the protein of hair
and wool. It soon became obvious that the a-helix is one of the fundamental folding
patterns of polypeptide chains.
The β-Pleated Sheet"A sheet of polypeptide chain formed by the folding back and forth of polypeptide chain is called β-pleated sheet.
A second common pattern in the secondary structure of proteins is the
β-pleated sheet (Figure).
In this structure, the chain is extended (as opposed
to coiled) and forms a zigzag pattern. The peptide backbones of neighboring chains
interact with one another through hydrogen bonding to form zigzag-shaped sheets.An antiparallel b-pleated sheet consists of extended polypeptide chains with
neighboring chains running in opposite (antiparallel) directions. In a parallel
b-pleated sheet, the polypeptide chains run in the same direction. Unlike the a-helix
arrangement, N—H and C=O groups lie in the plane of the sheet and are roughly
perpendicular to the long axis of the sheet. The C=O group of each peptide bond
is hydrogen-bonded to the N—H group of a peptide bond of a neighboring chain. Figure
 |
| β-pleated sheet conformation with three polypeptide chains running in opposite (antiparallel) directions.Hydrogen bonding between chains is indicated by dashed lines. |
As you study this section of b-pleated sheet, note the following:
1.The three polypeptide chains lie adjacent to each other and run in opposite (antiparallel) directions.
2. Each peptide bond is planar, and the a-carbons are trans to each other.
3.The C=O and N—H groups of peptide bonds from adjacent chains point at each
other and are in the same plane so that hydrogen bonding is possible between
adjacent polypeptide chains.
4. The R-groups on any one chain alternate, first above and then below the plane of
the sheet and so on.
The b-pleated sheet conformation is stabilized by hydrogen bonding between
N—H groups of one chain and C=O groups of an adjacent chain. By comparison,
the a-helix is stabilized by hydrogen bonding between N—H and C=O groups
within the same polypeptide chain.
Some proteins—such as silk—have the β-pleated sheet structure throughout their
entire chains. Since the protein chains in the β-pleated sheet are fully extended, silk is
inelastic. Many proteins have some sections that are β-pleated sheet, other sections
that are a-helical, and still other sections that have less regular patterns referred to as
random coils.